What makes species different? Why is it that many species of plant or animal can exist, in extremely close proximity, all competing for resources? The traditional view is that each species occupies its own ecological niche, defined as the set of responses to a species’ environment 1, including physiological, behavioural and reproductive distinctions. This means that species reduce competition by separation of their functional roles within the ecosystem.
However, some systems appear to contain far more species than would be expected from niche differences alone2. This begs the questions, what generates this biodiversity? A new theory of biodiversity attempts to explain this with the rather controversial assumption that species are ecologically identical: enter Neutral Theory.
Neutral theory’s origins
Stephen Hubbell first showed that models based on neutral principles could reproduce several notable biodiversity patterns 3 founded on ideas that stretched back as far as MacArthur and Wilson’s theory of island biogeography4. The original neutral model consisted of a very simple structure of two communities of individuals: one dynamic local community and one, usually much larger, metacommunity containing the full assemblage of possible species. The two basic steps of the original, spatially implicit model are
- All individuals in the community die.
- A replacement for each individual is randomly chosen either from the previous local community or, with some small probability, from the metacommunity as an immigration event.
This process is repeated many times, eventually producing a dynamic equilibrium in the local community where species go extinct at the same rate as immigration occurs from the metacommunity. As seen in Fig. 1, regardless of the starting conditions of species composition, the system approaches the same dynamic equilibrium (although it is worth mentioning that this can often take an extraordinarily long time).
Fig. 1: Species richness over time in a non-spatial neutral model.
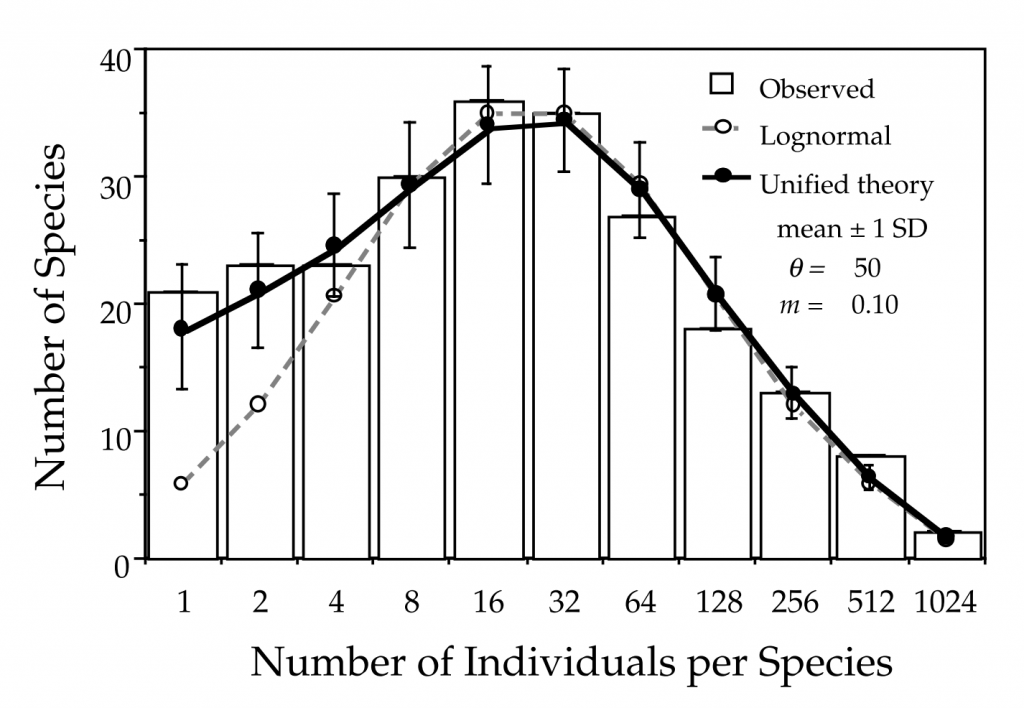
The metacommunity itself can be generated through a similar process of births and deaths, replacing the immigration with speciation, the generation of an entirely new species to the system. By modifying the parameters of immigration, speciation and the number of individuals in the metacommunity itself, the species richness (number of species), the species abundance distribution (SADs) and the species-area relationship (SAR) could be reproduced (Fig. 2).


A very simple simulation is shown in Fig. 3. The species richness decreases from around 4000 to approach a dynamics equilibrium of 5-10 species, but which species exactly make up these 5-10 species change over time (note the changes in the dominant colours).
Testing neutral theory
Hubbell’s original model spawned significant discussion focussing on three broad questions:
- Can neutral models reproduce realistic biodiversity patterns?
- If the patterns are realistic, is this simply a statistical anomaly and can any other models work similarly?
- Are diverse systems really dominated by neutral processes?
Since the turn of the century there have been a whole array of studies testing neutral theory in a wide variety of systems and on many metrics of diversity5,6. Some systems, particular coral reefs, seemed to oppose neutral theory7, but trees8, riverine systems9 and plankton10 all appear to display at least some element of neutrality.
There is still a huge amount of debate as to the utility of neutral theory11,12 particularly because many species are clearly not equivalent – no one would claim that a swan performs the same ecological role as a sparrow. With this in mind, is there a role for neutral theory despite its implausibility?
Neutral theory as a tool for ecology
One incredibly useful feature of neutral theory is that it can provide a baseline assumption for how dynamics unfold without additional effects, such as niche dynamics. When tested against real systems, this allows us to understand the relative contribution of other important processes, such as density dependence, competition and ecosystem structure.
Due to the relative simplicity of the model, it is possible to form equations that describe outcomes without requiring a full simulation. Within ecological theory, neutral models have been a powerful tool for improving our understanding13–18.
Neutral theory is highly scalable, due most to the advent of coalescence methods19 which massively reduce the computational cost of simulations. Neutral models provide one of the largest individual-based methods in ecology, opening up simulations to continental scales. The answer to why one would use neutral models in these circumstances is quite simple: there isn’t a good alternative!
Spatially explicit neutral theory
A major development for neutral models came with fully spatially explicit neutral theory. Here, individuals have a position in space and a dispersal kernel which describes how far individuals move from their parents. This is clearly much more realistic than the original model and also means aspects of habitat structure can be directly accounted for within the model.
A simple toy spatial model is shown in Fig. 4 using a map of Europe. Even this example has a few interesting features. Individual islands develop their own endemic species, which change over time as occasional speciation or immigration events take hold. Species on the mainland are more closely related (with more similar colours) than those on islands, yet there are more species within the same area on the continent than on an island. Occasionally the UK gets invaded by a species from Europe, which sometimes wins over the whole country. Spain, France and Italy often have considerably different species to central and eastern Europe.
Much more complex models can be developed under spatially explicit models, essentially allowing for any realistic landscape configuration to be explored. However, there appear to be severe issues with how realistic some of the patterns are from spatially explicit neutral models, particularly SADs20,21. It remains to be seen if these are due to elements of spatial structure not currently contained in neutral models, or if the fantastic fits from non-spatial models were simply coincidental.
The future of neutral theory
Protracted speciation
A obviously unrealistic feature of most neutral models is the “point speciation” process: individuals instantly become brand new species. In reality, speciation takes time. This can be represented by protracted speciation22, where a minimum number of generations must pass before lineages become distinct species. Protracted speciation provides a more realistic interpretation of the speciation process which could lead to new insights.
Nearly-neutral models and emergent neutrality
Several studies have tested how combined approaches that integrate niche and neutral processes produce better fits to real biological patterns than each one individually23–31. This opens up a new area of integrated biodiversity models that have enormous potential.
Conclusion
Although controversial, neutral theory remains perhaps the best tool for simulating very large-scale biodiversity models based on individual processes. The expansion of recent tools and new methods for testing and applying neutral theory mean that, even if the ecosystems are rarely dominated by neutral processes, it will remain an integral part of ecological theory.
References
- 1.Chase, J. M. & Leibold, M. A. Ecological niches : linking classical and contemporary approaches. 212 (University of Chicago Press, 2003).
- 2.Grubb, P. J. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52, 107–145 (1977).
- 3.Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography. Monographs in Population Biology 17, 375 (2001).
- 4.MacArthur, R. & Wilson, E. O. The Theory of Island Biogeography. 203 (Princeton University Press, 1967).
- 5.Rosindell, J., Hubbell, S. P. & Etienne, R. S. The Unified Neutral Theory of Biodiversity and Biogeography at Age Ten. Trends in Ecology & Evolution 26, 340–348 (2011).
- 6.Rosindell, J., Hubbell, S. P., He, F., Harmon, L. J. & Etienne, R. S. The case for ecological neutral theory. Trends in Ecology and Evolution 27, 203–208 (2012).
- 7.Dornelas, M., Connolly, S. R. & Hughes, T. P. Coral reef diversity refutes the neutral theory of biodiversity. Nature 440, 80–82 (2006).
- 8.Chave, J. & Leigh, E. G. A Spatially Explicit Neutral Model of b-Diversity in Tropical Forests. Theoretical Population Biology 62, 153–168 (2002).
- 9.Muneepeerakul, R. et al. Neutral metacommunity models predict fish diversity patterns in Mississippi-Missouri basin. Nature 453, 220–222 (2008).
- 10.Danino, M., Shnerb, N. M., Azaele, S., Kunin, W. E. & Kessler, D. A. The effect of environmental stochasticity on species richness in neutral communities. Journal of Theoretical Biology 409, 155–164 (2016).
- 11.Vergnon, R., Dulvy, N. K. & Freckleton, R. P. Niches versus neutrality: Uncovering the drivers of diversity in a species-rich community. Ecology Letters 12, 1079–1090 (2009).
- 12.Purves, D. W. & Turnbull, L. A. Different but equal: The implausible assumption at the heart of neutral theory. Journal of Animal Ecology 79, 1215–1225 (2010).
- 13.Alonso, D. & McKane, A. J. Sampling Hubbell’s neutral theory of biodiversity. Ecology Letters 7, 901–910 (2004).
- 14.Alonso, D., Etienne, R. S. & McKane, A. J. The merits of neutral theory. Trends in Ecology and Evolution 21, 451–457 (2006).
- 15.Green, J. L. & Plotkin, J. B. A statistical theory for sampling species abundances. Ecology Letters 10, 1037–1045 (2007).
- 16.Vanpeteghem, D. & Haegeman, B. An analytical approach to spatio-temporal dynamics of neutral community models. Journal of Mathematical Biology 61, 323–357 (2010).
- 17.Campos, P. R. A., Rosas, A., de Oliveira, V. M. & Gomes, M. A. F. Effect of Landscape Structure on Species Diversity. PloS one 8, e66495 (2013).
- 18.O’Dwyer, J. P. & Cornell, S. J. Cross-scale neutral ecology and the maintenance of biodiversity. Scientific Reports 8, 1–8 (2018).
- 19.Rosindell, J., Wong, Y. & Etienne, R. S. A coalescence approach to spatial neutral ecology. Ecological Informatics 3, 259–271 (2008).
- 20.Matthews, T. J. & Whittaker, R. J. Neutral theory and the species abundance distribution: Recent developments and prospects for unifying niche and neutral perspectives. Ecology and Evolution 4, 2263–2277 (2014).
- 21.Al Hammal, O., Alonso, D., Etienne, R. S. & Cornell, S. J. When Can Species Abundance Data Reveal Non-neutrality? PLoS Computational Biology 11, 1–23 (2015).
- 22.Rosindell, J., Cornell, S. J., Hubbell, S. P. & Etienne, R. S. Protracted speciation revitalizes the neutral theory of biodiversity. Ecology Letters 13, 716–727 (2010).
- 23.Gravel, D., Canham, C. D., Beaudet, M. & Messier, C. Reconciling niche and neutrality: The continuum hypothesis. Ecology Letters 9, 399–409 (2006).
- 24.Chisholm, R. A. & Pacala, S. W. Niche and neutral models predict asymptotically equivalent species abundance distributions in high-diversity ecological communities. Proceedings of the National Academy of Sciences 107, 15821–15825 (2010).
- 25.Barabás, G., D’Andrea, R., Rael, R., Meszéna, G. & Ostling, A. Emergent neutrality or hidden niches? Oikos 122, 1565–1572 (2013).
- 26.Pigolotti, S. & Cencini, M. Species abundances and lifetimes: From neutral to niche-stabilized communities. Journal of Theoretical Biology 338, 1–8 (2013).
- 27.Tang, J. & Zhou, S. Hybrid niche-neutral models outperform an otherwise equivalent neutral model for fitting coral reef data. Journal of Theoretical Biology 317, 212–218 (2013).
- 28.Noble, A. E. & Fagan, W. F. A niche remedy for the dynamical problems of neutral theory. Theoretical Ecology 8, 149–161 (2014).
- 29.Püttker, T., de Arruda Bueno, A., Prado, P. I. & Pardini, R. Ecological filtering or random extinction? Beta-diversity patterns and the importance of niche-based and neutral processes following habitat loss. Oikos 124, 206–215 (2015).
- 30.Godsoe, W., Jankowski, J., Holt, R. D. & Gravel, D. Integrating Biogeography with Contemporary Niche Theory. Trends in Ecology and Evolution 32, 488–499 (2017).
- 31.Godoy, O., Bartomeus, I., Rohr, R. P. & Saavedra, S. Towards the Integration of Niche and Network Theories. Trends in Ecology and Evolution 33, 287–300 (2018).